Elements That Have One Valence Electron Tend to
As it needs only one electron in its valence shell to complete the octet and attain the noble gas configuration of Argon left 288 right it readily accepts the electron given by the sodium atom. More energy levels B.

Section 3 Representative Groups Key Concepts Why Do The Elements In A Group Have Similar Properties What Are Some Properties Of The A Groups In The Ppt Download
That rule works in most cases if we only look at elements at the top of the various Groups in the periodic table.

. The three main groups of elements are metals nonmetals and. Are not solid at room temperature C. As you move down the same column of the periodic table elements have A.
The number of valence electrons in an atom governs its bonding behavior. They are more stable when they have 8 valence electrons so they want to lose that valence electron. Halogens have 7 valence electrons which explains why they are the most active non-metals.
The halogens all have the general electron configuration ns2np5 giving them seven valence electrons. But the rule breaks down. The elements of Group 17 fluorine chlorine bromine iodine and astatine are called the halogens.
Be highly reactive C. The elements in this family are fluorine chlorine bromine iodine and astatine. Alkali Metals Group 1 metals on the periodic table that contain 1 valence electron and lose their valence electrons the most easily making them the most reactive metals.
Which elements lose electrons most easily. That is a very rough and ready rule for the Main Group elements but cannot be relied upon. Which element has valence electrons in the n 2 shell.
Most elements on the left side of the periodic table are A. Generally elements in Groups 1 2 and 13 to 17 tend to react to form a closed shell with the electron configuration s²p⁶. 1 day agoThe greater the number of electrons an atom has to borrow or to lend the greater the activity of the atom.
The element has a full innermost electron shell of two electrons and a full shell of eight electrons in the next shell. Which group contains elements with two valence electrons. The number of electrons in an atoms outermost valence shell governs its bonding behaviour.
Group 17 elements form. The third shell which is the outermost and the valence shell has only one electron. Elements that have one valence electron tend to be highly reactive form ions become charged all of the above.
Have two valence electrons that form compounds with calcium and magnesium. Have really small atomic masses B. Number of valence electrons.
A sodium atom tends to lose one electron when it reacts true or false true. This is because such an atom has only a single valence. Atoms of group 18 elements have eight valence electrons or two in.
All of the above. A lower atomic number D. This makes more energy required being less reactive.
Elements that have one valence electron tend to A. Sodium has one valence electron. However element B has four valence electrons which should transfer or gain another four electrons to achieve stability.
They want to have a full octet so they are stable. Calcium is a group 2 element with two valence electrons. The most reactive kind of metallic element is an alkali metal of group 1 eg sodium or potassium.
Generally elements in Groups 1 2 and 13 to 17 tend to react to form a closed shell with a noble gas electron configuration ending in ns2 np6. All of the above ____ 15. Nonmetallic elements tend to have a positive valence and tend to be electron borrowers.
All of the above _____8. Answer 1 of 6. When they lose their 1 valence electron they have a full octet and are therefore stable.
Valence electrons influence chemical reactivity. The three main groups of elements are metals nonmetals and. They do this by bonding with other elements.
Does Period 4 have 7 valence electrons. The three main groups of elements are metals nonmetals and A. Elements whose atoms have the same number of valence electrons are grouped together in the Periodic Table.
Be highly reactive B. Have one valence electron that is easily removed to form a positive ion D. Alkali metals have 1 valence electron on their outer shell.
A different group number _____7. Elements that have one valence electron tend to a. Metals tend to have a negative valence and tend to be electron borrowers.
Elements that have one valence electron tend to A. The most reactive metals are those from Groups 1 and 2. Elements tend to react so that they acquire the electron structure of a halogen true or false false.
Elements whose atoms have the same number of valence electrons are grouped together in the Periodic Table. METALS The most reactive metals are. Each element in an element family shares the same A.
Elements that have one valence electron tend to. Elements in group 1 lose their one valence electron forming an ion with a 1 charge true or false true. Group 17 elements form a.
Therefore elements whose atoms can have the same number of valence electrons are grouped together in the periodic table of the elements. Element A has a valence electron which can easily give to another element to achieve stability. What elements have the valence electron configuration that is given by ns2.
Start studying the Life Science Chapter 9 Chemical Reactions flashcards containing study terms like A mixture can be distinguished from a pure substance because it can be separated by physical means The representative elements have one to eight valence electrons An atom becomes a positive ion by gaining an electron.
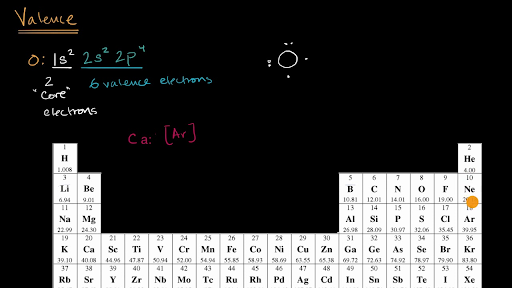
Valence Electrons Video Khan Academy

3 433 Gostos 24 Comentarios Vanessa Adagio Studies No Instagram Managed To Get Stuff Inspiracao De Estudo Organizacao De Estudo Motivacao Para Estudar

Chapter 5 Periodic Table H Li Na K Rb Cs Fr Be Mg Ca Sr Ra Ba F Cl Br I Uus At O S Se Te Uuh Po N P As

Periodic Table Groups Graphic Organizer Personalities Distance Learning Teaching Chemistry Homeschool Science Teaching Science

Valence Electrons Ck 12 Foundation

Lesson Explainer Electronic Configurations Of Transition Metals Nagwa

Ions Present In Compounds Composed Of Elements With Very Low Very High Electroneg Ie Metals Nonmetals This Ionic Bonding Covalent Bonding Chemical Bond

Chemistry For Kids Chemical Bonding Chemistry For Kids Covalent Bonding Chemistry

Periodic Table Groups Graphic Organizer Personalities Distance Learning Informational Texts Activities Science Teaching Resources Graphic Organizers
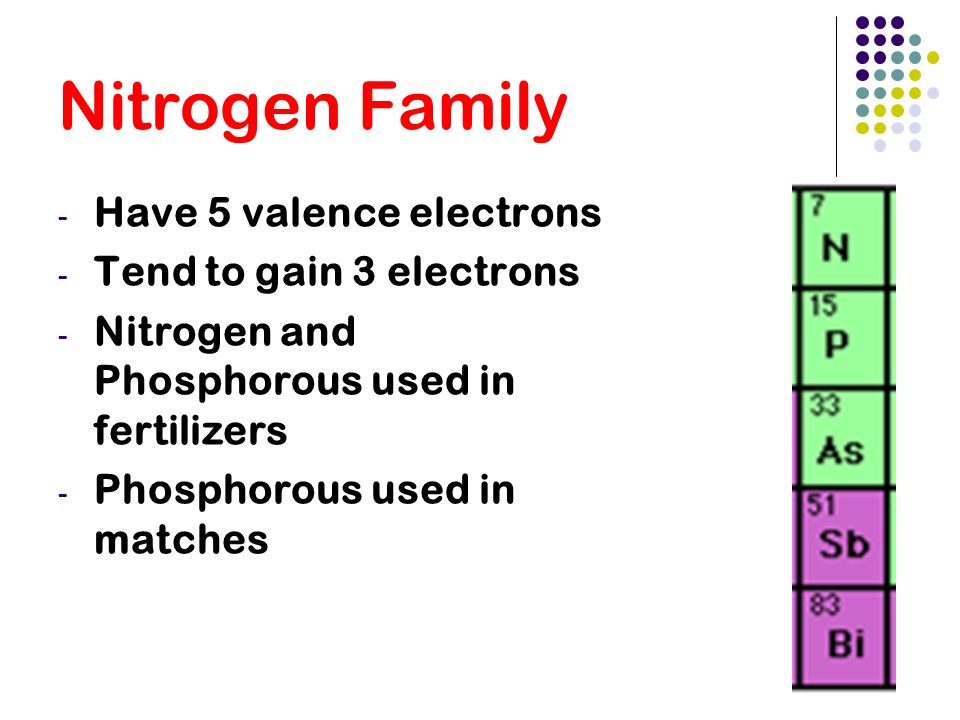
Chapter 5 The Periodic Table Ppt Video Online Download

Pin By Aguerra On Chemistry Chemistry Positivity Bullet Journal

Valence Electrons Ck 12 Foundation

Periodic Table Chapter 6 Periodic Table Many Different Versions Of The Periodic Table Exist All Try To Arrange The Known Elements Into An Organized Table Ppt Download

Chapter 6 The Periodic Table Ppt Download

Ewg V Edg Organic Chemistry Organic Chem Electron Donating Groups
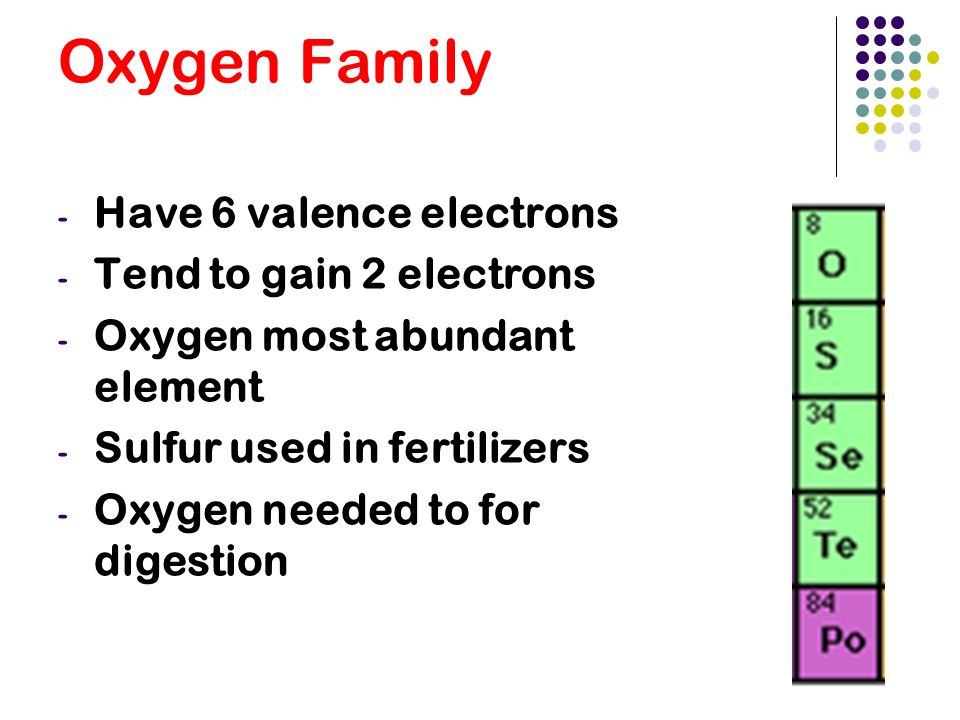
Chapter 5 The Periodic Table Ppt Video Online Download

Tips For Scaffolding In The Chemistry Classroom Science And Math With Mrs Lau Teaching Chemistry Chemistry Lessons Chemistry Classroom

Physical Science Speedy Midterm Review Ppt Download

Yesterday For English We Went On A Field Trip I Haven T Been On One Of Those In Over 5 Years The Usual Notes Inspiration Study Notes Good Notes

Comments
Post a Comment